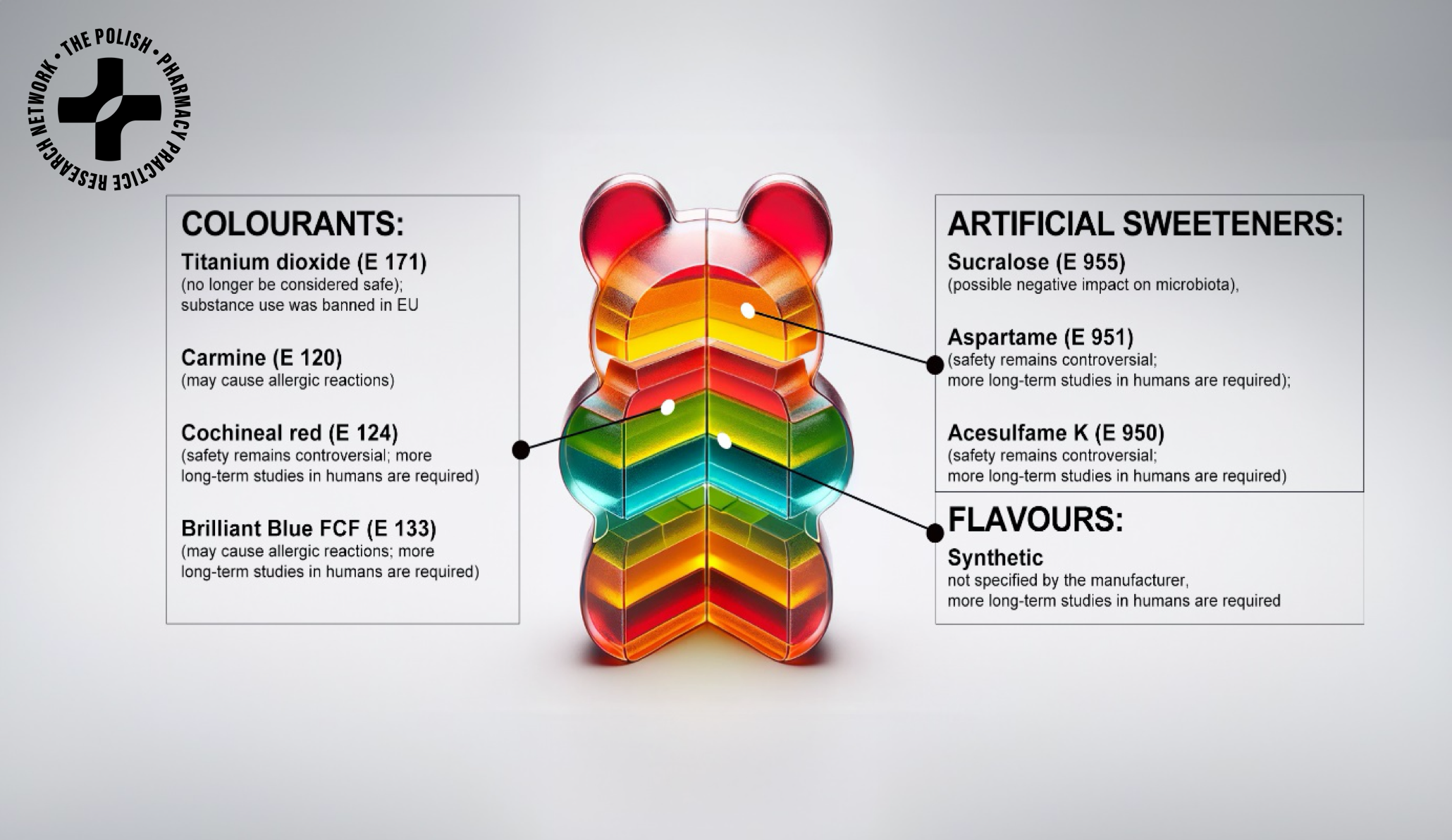
Gummy dietary supplements for children have gained widespread popularity as a convenient and attractive way to support nutrition. However, this trend raises serious concerns about their composition, regulation, and safety—especially when intended for the youngest consumers. In this study, we actively involved both pharmacists and clinical dietitians to comprehensively evaluate the declared content of 103 gummy supplements available on the Polish market.
The multidisciplinary team analysed ingredients such as sweeteners, gelling agents, flavourings, and colouring substances. The results revealed a high content of simple sugars (e.g., glucose syrup, sugar), artificial sweeteners (e.g., sucralose, acesulfame K), and synthetic colourants (e.g., Brilliant Blue FCF, carmine, cochineal) in many products. Such additives may pose risks to children’s health—affecting metabolic processes, gut microbiota, or even behaviour.
The study highlights a significant regulatory gap. Unlike infant foods, dietary supplements for children are often insufficiently controlled and marketed without adequate safety evaluation. This situation exposes children to potentially harmful substances with limited parental awareness.
Our conclusion is clear: there is an urgent need to develop and implement evidence-based, age-specific regulatory standards for dietary supplements aimed at children. By involving pharmacists and dietitians in this process, we ensure a balanced approach that protects health while supporting rational supplementation practices.